Mitigating Corrosion in Caustic Vessels
Causes of Caustic Corrosion in Demercaptanization Processes
Caustic conditions, within MEROX and other demercaptanization processes, can rapidly attack the steel substrate’s protective magnetite layer and cause metal wastage. Following the destruction of the protective magnetite layer, NaOH can additionally react with exposed elemental iron to form atomic hydrogen, leading to hydrogen embrittlement, decreased wall thickness, and caustic stress corrosion cracking (CSCC) or caustic embrittlement. These phenomena have led to multiple plant failures under different caustic conditions.

Caustic Corrosion in High pH Vessels
Caustic stress corrosion cracking, aggressive pitting corrosion, and caustic embrittlement are common problems within extractors, absorbers, settlers and other High pH columns and vessels. Typically used within the Demercaptanization units, including Merox, these vessels generate sweetened gas condensate products of much higher quality and added value.
Example: Stress Corrosion Cracking Caused by Caustic Embrittlement
As seen in Figure 1, caustic stress corrosion cracking caused by caustic embrittlement resulted in the failure of an H2S absorption vessel due to cracks at the main vessel nozzle. The vessel contained a 20% solution of potassium hydroxide (KOH), potassium carbonate (K2CO3) and arsenic at 6550 kPa (950 psi) and operated at 33°C (91°F). Micrographs identified cracking indicative of caustic embrittlement caused by KOH at the failure locations.
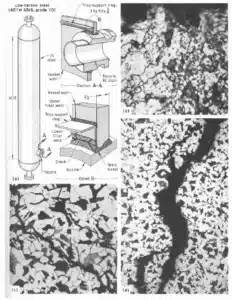
Example: Catastrophic Failure of a High-Pressure Vessel Due to Caustic Corrosion
In a 2009 case study, caustic stress corrosion cracking from a 4% Sodium Hydroxide (NaOH) and water solution caused the catastrophic failure of a high-pressure vessel manufacturing quartz crystals in the USA. One fatality and several injuries occurred. Micrographs of the failed vessel confirmed that CSCC was the likely failure mechanism. Caustic stress corrosion cracking was later found in multiple pressure vessels throughout the facility.

Caustic Corrosion: Material Selection
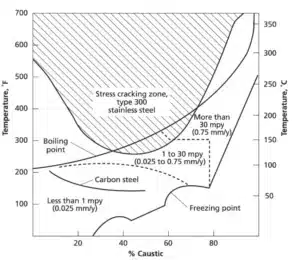
Material susceptibility to caustic corrosion and CSCC is heavily dependent on the caustic concentration, process temperature, and metal alloy type. Carbon steel is the most common material used for caustic environments and is susceptible to CSCC and general corrosion or pitting at temperatures above 50-60°C and NaOH concentrations below 20 wt. %. Stainless steel provides more protection but is at risk of CSCC and aggressive corrosion above 120°C.
Solutions to Metal Wastage Caused by Caustic Corrosion
Several solutions have been tried with varying degrees of performance in addressing caustic corrosion, including vessel replacement, organic coatings and linings, weld metal overlay, including Monel and Hastelloy, and modified HVTS® Metalspray® cladding. Each caustic service material selection solution carries its pros and cons.
Caustic Vessel Replacement due to Corrosion
- Pro: Complete replacement allows for a more appropriate material selection within the new vessel for the environment.
- Con: This solution is very expensive, has a significant lead time, requires a lot of forward planning, typically involves a complex removal and installation program to locate and tie-in the new vessel as well as contingency plans for delays in installation. The new vessel may experience the same problems, shortening its expected asset life
Organic Coatings and Linings
- Pro: Low cost, temporary solution, applied during a planned turnaround. There is some success of organic coatings performing well in acidic service.
- Con: Short coating life, with multiple in-service failures reported due to the aggressive and oftentimes unpredictable nature of the caustic environment. Once the coating fails, the metal loss will continue, potentially leading to failing the fitness for service assessment, and requiring a forced shutdown.
Weld Metal Overlay, Monel and Hastelloy
- Pro: Established solution in amine and other aggressive environments, where high temperatures and pressures exacerbate the corrosive nature of the process media.
- Con: Field application is costly and slow, with low coverage rates typically requiring an extended project critical path. Welding creates HAZ (Heat Affected Zones) requiring equipment stabilization and PWHT (Post Weld Heat Treatment), making field welding solutions riskier, expensive and less cost-effective.
Modified HVTS Metalspray®℠ cladding
- Pro: Established solution in amine and other aggressive environments, where high temperatures and pressures exacerbate the corrosive nature of the process media. Cost-effective when compared to weld overlay due to faster application, shortening the critical path and thus cutting down the turnaround days. Positive references from National and International Oil Companies confirm HVTS® Metalspray® cladding as a suitable corrosion protection method.
- Con: Unmodified Monel and Hastelloy claddings are more permeable if applied by thermal spray methods rather than welded. Service provider selection plays a crucial role in the success of field applied thermal spray application. The thermal spray provider must present evidence that their material, conveyancing technology, and application methodology are suitable for the in-service environment.
HVTS® Alloy Cladding Suitability for Caustic Environments
A Solution for Caustic Corrosion Mitigation
First presented at the Materials Performance & Welding Technologies NACE Conference in 2019, paper number MPWT19-14436, “Mitigation of Caustic Corrosion: Alloy and Process Considerations for High Velocity Thermal Spray Cladding” discusses the suitability and performance of modified HVTS® applied alloys for service where high pH general corrosion or caustic cracking (CSCC) may occur.
Extensive testing has been undertaken in both ambient and high pressure and temperature autoclave conditions to better understand material performance in caustic environments.
While Nickel Alloy 200 and Monel 400 may be deemed appropriate based on traditional material selections, thermal spray process considerations in the material deposition and the impact of ancillary elements in the process stream, such as halides, render these alloys unsuitable.
More complex Nickel alloy cladding systems were evaluated in this study with suitable material recommendations, applied without the heat impact of welding or to protect surfaces, where heat-affected zones (HAZ) have been created and post-weld heat treatment is problematic.
Caustic Corrosion HVTS® Study – Test Results and Conclusions
No iron-form corrosion products were seen on the surface of the exposure test area, and the HVTS® systems were completely intact. Additionally, SEM imaging and EDS elemental analysis performed on the test panels confirmed that no corrosion product was present at the sample coating-substrate bond line interface or within the thermal spray matrixes.
Bulk elemental scans were also performed to detect anomalies in the thermal spray matrix or the presence of test solution elements such as Sodium (Na), which may have penetrated the cladding and could indicate potential corrosion pathways. Scans of the conventional NiCrMo control system did show elemental Sodium (Na) in the uppermost layer of the thermal spray system; this could indicate eventual caustic penetration of non-modified alloy systems. All modified NiCrMo-XX thermal spray systems prevented caustic penetration, exposure, and corrosion of the carbon steel substrate.
Corrosion risks during the Merox process
During the Merox process, mercaptans are oxidized to disulfides using an oxidizing agent such as hydrogen peroxide. While the chemical reaction effectively eliminates mercaptans, Merox corrosion is the byproduct of the process, particularly in the form of organic acids such as acetic acid and formic acid.
When acidic byproducts attack the metallic surfaces of equipment used in the Mercaptan Oxidation process, the Merox corrosion that occurs can lead to structural damage in Merox caustic vessels.
As with all demercaptanization processes, it is essential to manage corrosion risks during the Merox process to safeguard the performance and lifetime of the asset.
How does IGS mitigate Merox Corrosion?
IGS protects Merox Caustic vessels from corrosion around the world thanks to its High Velocity Thermal Spray (HVTS®) solution. Field applied, HVTS® high nobility alloy cladding provides an effective protective barrier for caustic vessels hosting aggressive corrosion environments.
Case Study: HVTS Prevents the Need for Vessel Replacement
IGS delivers effective caustic corrosion mitigation
A major chemicals producer with production facilities in the USA and the Middle East was experiencing severe corrosion issues in their caustic process vessels.
After repeated failures of organic coatings and the resulting ongoing substrate metal wastage, the client was considering replacing the vessels.
Upon learning about IGS HVTS® high nobility alloy cladding as a field applied, effective corrosion barrier technology for caustic service, the asset owners commissioned additional long-term testing, replicating their operating service conditions, to further validate the solution suitability for their process. The result of the testing was conclusive, in that IGS modified NiCrMo-XX alloys provide an effective protective barrier for the vessel carbon steel substrate in the client-specific aggressive caustic environments.
Field Application of the HVTS® Alloy Cladding for Caustic Service in the Middle East Prevents Caustic Stress Corrosion Cracking
The in-situ application was carried out in early Spring 2020 with 351m2 of IGS HVTS® cladding applied to a Caustic Wash Settler in the Middle East to prevent stress corrosion cracking in the vessel shell and weld seams caused by the corrosive caustic environment.
The original process critical vessel was installed and commissioned in 2011. Following a significant failure, the Caustic Settler was replaced in 2015. An organic lining was installed to the entire internal area of the vessel. Repeated failures of the organic coating over the years followed, leading to a progressive loss of metal wall thickness.
Following conclusive HVTS® test results in 2019, the asset owner partnered with IGS to install the HVTS® noble alloy barrier to clad the entire internal shell and prevent further metal wastage, safeguarding the vessel’s integrity for the future.
The Maintenance Manager at the Middle East Chemicals facility commented: “We are extremely satisfied with the HVTS® technology’s performance results and operational capability of the IGS installation teams on-site.”
A similar application is due to take place in one of their US facilities shortly.
READ: Organic Coatings in Pressure Vessels: Internal Coating Failure Analysis
Refinery Success Story:
HVTS® Cladding Combats High Corrosion Rate in a Caustic Pressure Vessel
Jason Lynn, IGS Director of Business Development, shares this success story from a Gulf Coast refinery, where the project not only stopped corrosion in a caustic pressure vessel but also delivered substantial cost savings.
Our unique High Velocity Thermal Spray (HVTS®) coating supported enabled the client to keep this piece of equipment in service for a longer period of time. In terms of dollars, you could quantity that in turnaround time and materials to be in the span of a few million dollars per downtime. Our HVTS® project allowed them to go multiple turnarounds, making it a really impactful solution for the client.
HVTS® Alloy Cladding for Mitigating Corrosion in your Caustic Environment
We protect Merox Caustic vessels around the world. To ensure the suitability of the HVTS® alloy cladding for your specific environment, we welcome you to fill in the form below. Your local subject matter expert will be in touch shortly to help with your asset integrity. More laboratory testing may be required, which can be completed promptly in the IGS Technology Solutions Laboratory.

I’m here to help
Colin Bateman
IGS Subject Matter Expert
Free consultation with an IGS Subject Matter Expert
IGS is here to provide information, answer questions and create an effective solution for your needs.
